The Market
The urinary catheter market is worth
~$1bn p.a. and within this market some sectors are increasing in value by more
than 10% CAGR. The insertion of any catheter into the body is a potential route
for infection by the very nature that they are in contact with the outside
world; additionally urinary catheters carry a concomitant risk to encrustation
leading to blockage and catheter associated urinary tract infection (CAUTI)
which increases significantly with the indwelling time of the catheter (+5% per
day). CAUTI accounts for >40% of all institutionally acquired infections.
The Foley catheter was first introduced
in the mid-1930s and is the most common form of urinary catheter in use today.
It has undergone a series of incremental changes resulting in the modern
version, predominantly through a change in materials and lubrication
technologies for patient comfort.
The Foley catheter however is
implicated in 80% of the CAUTIs noted above.
Current solutions in catheter technology
provide for antibiotic impregnated devices and silver ion coating technologies.
These devices have been widely adopted, but the data available now do not fully
support the claims associated with these technologies and bacterial
antimicrobial resistance to these approaches has been noted as a potential
problem due to the selective pressure induced by the nature of their action.
There are significant market drivers to provide improved solutions to CAUTI, the
treatment of which adds a huge burden on the health service ($1,000 per
patient). These costs are no longer reimbursable under the Medicare program in
the US.
Technical
Information
We are producing materials that have
intrinsic anti-adherence properties on which bacteria find it difficult to
attach and grow. In screening against a range of pathogens (uropathogenic
Escherichia coli, Pseudomonas aeruginosa and
Staphylococcus aureus) the large number of polymers screened have
demonstrated a wide range of bacterial adherence characteristics (Figure 1).
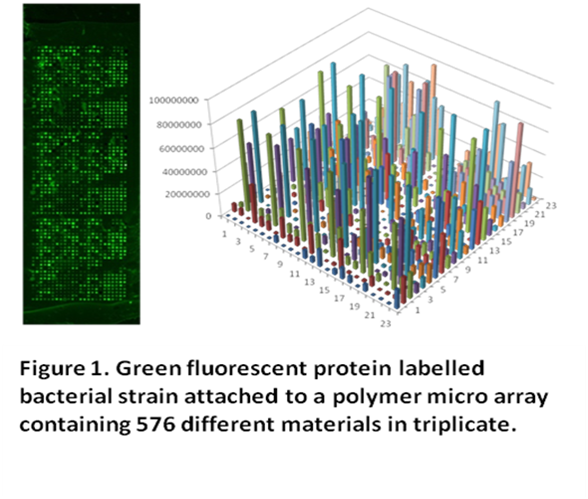
A total of 22 candidate polymers have
been selected that show significant reduction in bacterial adherence. These
polymers are the subject of scale-up protocols indicating significant improved
performance against uncoated silicone catheters and the market leading anti
infective coating Bactiguard® (Figure 2). Candidate polymers are being further
characterised and evaluated for their suitability in production and/or as
coating technologies.
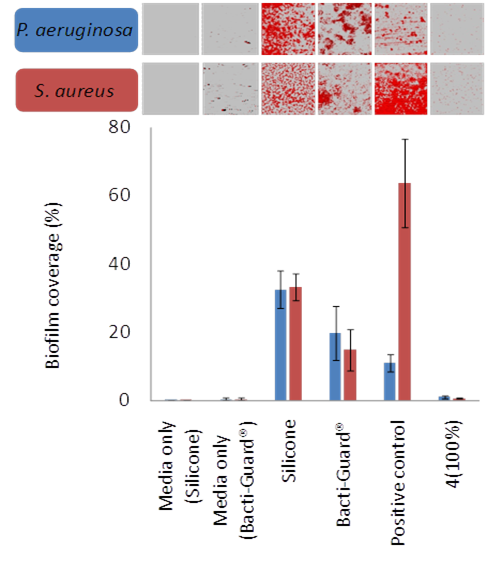
Figure 2. Comparison of bacterial coverage for PA
and SA on a hit polymer (4 100%), uncoated silicone catheter, a commercial
catheter (Bacti-Guard) and a positive control with the negative media-only
controls.
In vitro studies have demonstrated the
rationale and predictable antibacterial properties of the candidate polymers.
Scale-up onto silicone catheters have resulted in excellent in vivo results from
a murine subcutaneous foreign body model shown in Fig 3.
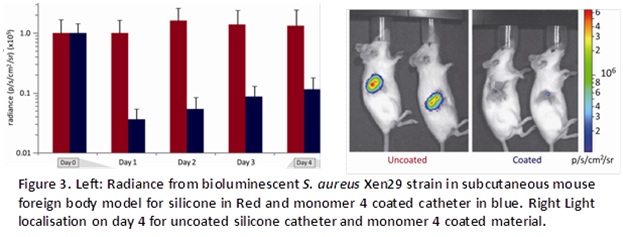
This illustrates clearance of a
bacterial inoculate by the host immune defence system when placed in
the lead polymer in vivo, compared to the silicone catheter where bacteria
adhere, form biofilm and persist over the 4 day period of the experiment.
We are undertaking a 4 year program to
evaluate this polymer coating technology and aim to license the technology
to key businesses that provide urinary catheters throughout the world
markets. We are not seeking to make incremental changes in the
performance of these devices, rather we are gearing up to provide step
change improvements over the existing competition.
Our planned route to market for the
technology is through the catheter market via a licensing deal with an
established supplier of urinary catheters prior to diversification into other
applications for the technology.
Patents
The technology employed is
protected by two patent applications which are being prosecuted internationally:
-Polymer arrays for biofilm
adhesion testing, publication number WO
2009/019519, 2007.
(http://uon.technologypublisher.com/technology/8194)
-Novel polymers which resist
bacterial attachment, UK Patent Application no: 1107416.8, 2011.